What is Hypochlorous Acid Water?
Table of contents
 Why hypochlorous acid now?
Why hypochlorous acid now?
 How does it work?
How does it work?
 Its power to kill pathogens
Its power to kill pathogens
 Its safety
Its safety
 Precautions for handling it
Precautions for handling it
 Effective storage period and storage method
Effective storage period and storage method
 Its manufacturing equipment
Its manufacturing equipment
Hypochlorous acid water - why is it attracting attention now?
The expansion of CoVID-19
When the CoVID-19 virus, SARS-CoV-2, began to rage in early 2020, the alcohol disinfectant, which was well known for its ease of use in medical facilities, resulting in a great deal of turmoil.
In such a circumstance, a reputation that "hypochlorous acid water (HAW) is usable as antiseptic solution" has spread. There were positive opinions about this matter as "It is excellent" and negative opinions as "I doubt it works." And this controversy involved national research institutes, universities, manufacturing companies, and even ordinary citizens. At present, the argument itself seems to have settled down, with the final report by the National Institute of Technology and Evaluation of Japan(NITE) that "HAW is effective against CoVID-19 when used correctly."
However, if you search on the internet there are many products named "hypochlorous acid ...", and the explanations for each are slightly or significantly different. When examined in detail, products with the same name as "hypochlorous acid water" may differ considerably in the ingredients, efficacy, safety, and usability. In addition, there often are inaccurate product descriptions about them that make you want to be angry from a scientific point of view.
We are selling a type of HAW named "CLEAN·REFRE." So we have to explain to our customers what HAW is. In the following sentences, we will explain the effects, precautions for handling, manufacturing methods of HAW from a scientific point of view based on the research results of universities, institutes, and us.
Many people may think that "I have heard the name hypochlorous acid water, but I don't know what it is. " Therefore, please forgive us for starting with a rudimentary explanation.

How does hypochlorous acid water work?
Hypochlorous acid
We refer to water that contains salt as "saltwater". Similarly, we refer to water that contains hypochlorous acid(HClO) as "hypochlorous acid water." You may have seen the actual hydrochloric acid and citric acid in your chemistry class, but haven't you seen hypochlorous acid? We make hydrochloric acid by dissolving hydrogen chloride (HCl) in water. If an oxygen atom (O) gets between the hydrogen (H) and the chlorine (Cl) of the hydrogen chloride molecule, a hypochlorous acid molecule is formed (see the figure below).
Fig.1: Structural formula and space-filling model of hypochlorous acid.
White is hydrogen, red is oxygen, and green is chlorine atom.
pm (picometer) means 1 x 10-12 meters. Source: Wikipedia Hypochlorous acid
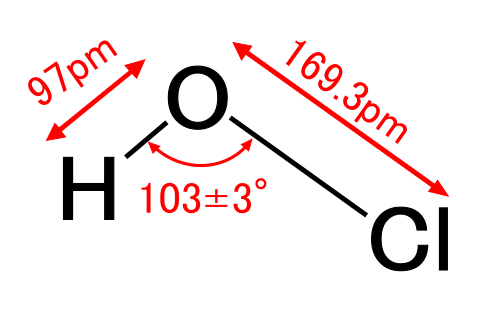
If you replace the chlorine atom in the above figure with a hydrogen atom, it will be in the same order as the molecule of water (H2O). A water molecule is composed of one oxygen atom that attracts electrons and two hydrogen atoms that repel electrons. Chlorine is essentially an atom that attracts electrons in a molecule such as HCl. But, in the hypochlorous acid molecule, it seems to play a role similar to a hydrogen atom in a water molecule. Being in such a position is unreasonable for the chlorine atom. Therefore, as will be explained later, hypochlorous acid molecules are so unstable and fragile that they can exist for up to a few months in a sealed storage container put in a cold and dark place, but decompose and disappear in the air in a few hours. However, its fragility is the source of the powerful destructive ability of hypochlorous acid. Neutrophils (a type of white blood cell) in our body produce hypochlorous acid and use it to attack pathogens such as bacterias and viruses. Please read this report as an example.
The Japanese Waterworks Law stipulates that "chlorination should be performed so that free residual chlorine concentration is at 0.1 mg/L*1 (0.4 mg/L in the case of combined residual chlorine) or more." The "free residual chlorine" means "remaining free available chlorine." In this case, free available chlorine*2 is a sum of hypochlorous acid (HClO) and hypochlorite ion (ClO-).
*2 Chlorine in chlorine compounds that can cause strong reactions such as sterilization and bleaching is called "free available chlorine(FAC)" or "effective chlorine. " In the case of hypochlorous acid water, it is the total ability to oxidize the reaction partner for three types of compounds: hypochlorous acid molecule, hypochlorite ion, and chlorine molecule. FAC concentration is defined as the concentration of chlorine molecules that have the same oxidizing power as the total chlorine compounds. There are other chlorine-containing components such as hydrogen chloride and sodium chloride in hypochlorous acid water, but chlorine in these components is not considered to be FAC. Therefore, the "free available chlorine concentration" and "chlorine concentration" of hypochlorous acid water are different.
Oxidizing power of hypochlorous acid
The effectiveness of hypochlorous acid is exerted by oxidizing the attack target. When hypochlorous acid comes into contact with an organic substance that easily receives oxygen, it decomposes as follows: HClO → HCl + O and causes a reaction that forcibly gives oxygen to the substance. As a result, hypochlorous acid disappears, and a part of the organic substance is destroyed by oxidation. Parts of living organisms such as pathogens, and organic molecules such as alcohol are relatively easily oxidized by hypochlorous acid. In other words, hypochlorous acid is consumed by reacting with alcohol. On the other hand, plastics used for liquid containers are not easily oxidized or decomposed when they come into contact with hypochlorous acid water. Therefore, hypochlorous acid water in a PET bottle will not lose its effectiveness immediately.

The power of HAW to kill pathogens
Hypochlorous acid efficiently destroys minute pathogens
Because the hypochlorous acid molecule is electrically neutral (uncharged), it can cross the bacterial cell membrane and attacks essential elements such as the nucleus inside the cell (see below). Since it magically passes through the walls and directly hits important parts inside, the bacteria can't hold up at all.
Fig.2: Hypochlorous acid can cross the cell membrane and destroy inside the cell.
Modified after Nakamura et al.(2017)
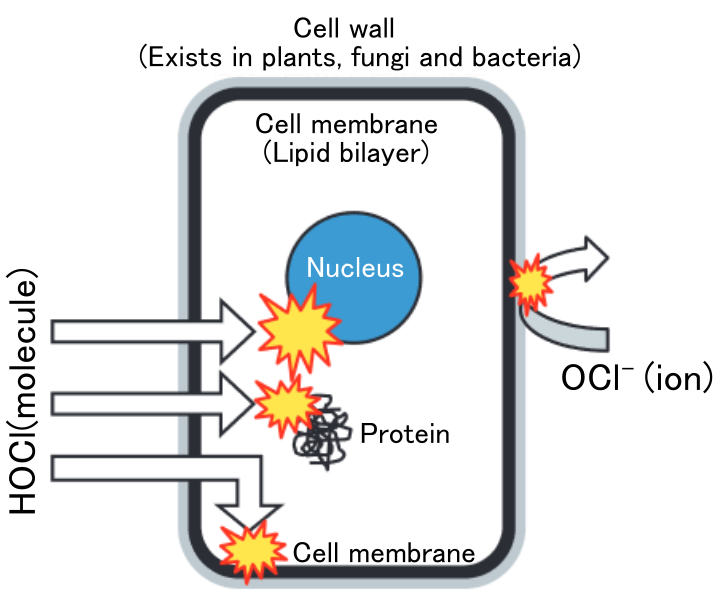
On the other hand, the active ingredient in sodium hypochlorite (NaClO) solution is hypochlorite ion (ClO-). The ion is negatively charged and cannot pass through the cell membrane. Therefore, it must destroy cell membranes to kill microorganisms, which requires high concentrations of ClO- ions. They say that the bactericidal activity of hypochlorous acid is 80-100 times stronger than that of hypochlorite ion(e.g. J. Japakaset et al, 2006 ). As a hypochlorous acid molecule goes inside cells and attacks key points, it is far more efficient than a hypochlorite ion.
The table below shows that the effect of hypochlorous acid water (FAC 40mg/L) on various pathogens is higher than the effect of sodium hypochlorite solution (FAC 1,000mg/L).
Table 1: Sterilizing activity of hypochlorous acid water.
Source: Fuctional Water Research Promotion Foundation in Japan
| Microorganisms / viruses | Hypochlorous acid water(40mg/L) | Sodium hypochlorite solution(1,000mg/L) | |
|---|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | ◎(10sec.) | ◎(10sec.) |
| MRSA (Methicillin-resistant Staphylococcus aureus) | ◎(10sec.) | ◎(10sec.) | |
| Bacillus cereus | △(3〜5min.) | △(3〜5min.) | |
| Mycobacterium tuberculosis | △(2.5min.) | ▲(30min.) | |
| Other acid-fast bacilli | △(1〜2.5min.) | ▲(2.5〜30min.) | |
| Gram-negative bacteria | Salmonella Enteritidis | ◎(10sec.) | ◎(10sec.) |
| Vibrio parahaemolyticus | ◎(10sec.) | ◎(10sec.) | |
| Escherichia coli O157:H7 | ◎(10sec.) | ◎(10sec.) | |
| Campylobacter jejuni | ◎(10sec.) | ◎(10sec.) | |
| Pseudomonas aeruginosa | ◎(10sec.) | ◎(10sec.) | |
| Virus | Norovirus (Feline calicivirus) | ◎ | ○ |
| Influenza virus (including H1N1pdm09) | ◎(10sec.) | ◎(10sec.) | |
| Enterovirus | ◎(10sec.) | ◎(10sec.) | |
| Herpes virus | ◎(10sec.) | ◎(10sec.) | |
| Fungus | Candida albicans | ◎(10sec.) | ◎(10sec.) |
| Aspergillus niger | △(5min.) | x(120min.) | |
| Penicillium cyclopium | △(5min.) | x(120min.) | |
An image of hypochlorous acid attacking viruses
Despite its powerful destructive power, a single hypochlorous acid molecule releases only one oxygen atom, so it can destroy only a small part of the pathogen. Inactivating viruses or bacteria that are much larger than the molecule requires a reasonable amount of hypochlorous acid, which means that HAW with a FAC concentration is ineffective. In fact, according to the report of NITE, hypochlorous acid water with a FAC concentration of less than 35 mg/L* was not sufficiently effective against SARS-CoV-2. Now, imagine a scene in which hypochlorous acid water with a FAC concentration of 50 mg/L fights against the SARS-CoV-2 in saliva, which is the main factor in transmitting the disease.
They believe that 1 mL of saliva from patients who can infect others with CoVID-19 contains about 1 million viruses. When the viruses are evenly distributed in a volume of 1 mL, the average spacing is 0.1 mm (100,000 nm). This distance is 1,000 times the size of the virus (about 100 nm). If you expand the virus to the size of a human with a height of 1.7 m, the distance to the nearest neighbor is 1.7 km. It is said to avoid "three dense states" to prevent CoVID-19 infection, but the virus itself does not seem to be dense. By the way, according to our calculation, there are 4.25 x 1017 hypochlorous acid molecules in 1 mL of HAW with a FAC concentration of 50 mg/L. Assuming that the hypochlorous acid molecules that can attack the virus are within 10 nm from the virus surface, the number of molecules within that range is approximately 300. If one-third of the molecules sneak inside the virus and destroy 100 sites, it must be sufficient to inactivate the virus. When the FAC concentration is 0.5 mg/L (about the concentration of tap water), there are only three hypochlorous acid molecules in the same area. Thus it will be difficult for them to fight the virus, which is 500 times larger than the molecule.
The difference in body size of 500 times is about the same as that between humans and gnats. Imagine if gnats attack you. It is awkward if three gnats attack you, but they will not be able to kill you. But, though it never happens in reality, if hundreds of gnats enter your body and attack your internal organs, the consequences will be horrifying. It will happen when hypochlorous acid attacks pathogens.

Safety of hypochlorous acid water
Yes, it is safe
Many papers have reported on the safety of HAW(e.g. L.Wang et al., 2007), and Japan Food Safety Commission described in the evaluation report for hypochlorous acid water (January 2007) as follows(The original text is in Japanese):
Please note that this evaluation report is about HAWs that meet the standards as food additives specified by the Ministry of Health, Labor and Welfare of Japan(MHLW).
Fig. 3: Composition ranges of HAWs defined as food additives for cleaning by MHLW, and of CLEAN·REFRE.
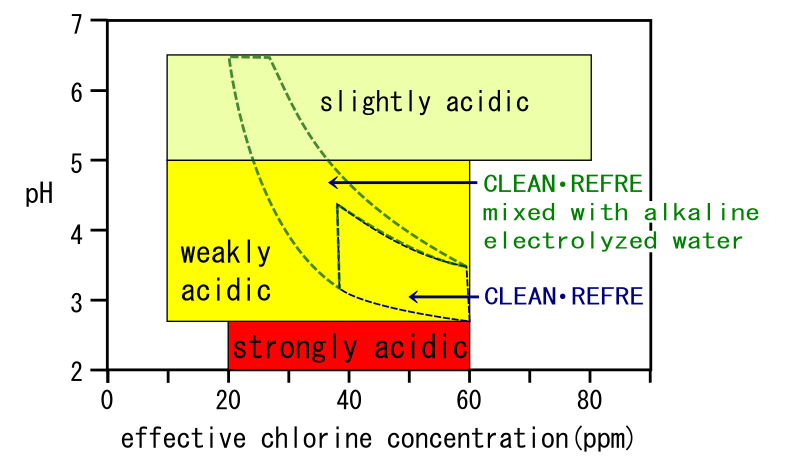
According to the regulations of MHLW, HAWs as food additives must meet the FAC concentration ranges and pH ranges shown in the above figure. It is stipulated that the strongly acidic and weakly acidic HAWs are produced by electrolysis of aqueous solutions of sodium chloride, and the slightly acidic HAW of hydrogen chloride with or without additive sodium chloride. The concentration ranges of these raw material aqueous solutions are also stipulated in the regulations. Even when a product is sold under the name of "hypochlorous acid water", if it is not manufactured in the manner specified by the regulations, it cannot be used as a food additive and its safety must be judged unconfirmed.
One more thing to write here is that the MHLW´s standards of the HAWs provide nothing about NaCl concentration in HAWs. Therefore some HAWs on the market possibly contain about 0.2wt% NaCl, even if they conform to the standard. Such products possibly accelerate metal corrosion where they are poured.
Spraying HAW
As the infection of CoVID-19 spread, the pros and cons of aerial spraying of HAW became a hot topic, and much discussion was held.
We don't think anyone argues that increasing the humidity in the indoor air by spraying freshwater is effective as a preventive measure against CoVID-19 and influenza infections. It is possible to reduce the FAC concentration as much as possible by diluting HAW with water. If, for example, spraying hypochlorous acid water with a FAC concentration of 60 mg/L into the air is harmful, how low is the FAC concentration to be harmless? Does spraying of the harmless HAW does not affect the virus? If so, it would be meaningless to spray HAW in the air …
The answer to such a question has not yet been clearly shown. It is a plausible opinion that it is safer not to do HAW spraying until its safety has been completely proven. However, some people argue that it is safer to spray HAW if a dangerous virus is around you. The same is true for the CoVID-19 vaccine, which has been put into use after an unprecedentedly short period of safety testing.
What we need to do now on these issues is to quickly collect reliable data so that we can accurately compare both the benefits and the risks to determine whether or not to use them. If the HAW concentration range, spray amount, and spray method that are harmless to the human body and can inactivate viruses in the air are clarified, the spraying will be a powerful means to prevent many types of infectious diseases.
WHO warned that it is dangerous to spray disinfectants in the air, probably because the actual health hazards of air sprays have been reported. However, it is something about a common disinfectant other than HAW, and WHO has not confirmed the dangers of HAW spraying. And at least in Japan, HAW is not treated as a disinfectant.
We have never seen any data showing that spraying hypochlorous acid water(not sodium hypochlorite water) in the air is dangerous to the human body. Many documents support the safety and effectiveness of HAW, including its aerial spraying. For example, the HAW Promotion Council in Japan has compiled materials(A Japanese page. Sorry).

General precautions when handling hypochlorous acid water
We have mainly explained the good points of HAW, but there are some things to be careful about when using HAW.
(1) Do not confuse HAW(HClO) with sodium hypochlorite(NaClO). They are completely different, the latter being harmful to the human body at normal use concentrations
(2) Even products sold under the name of "hypochlorous acid water" may have different compositions, and may contain extra ingredients. We recommend that you use HAW manufactured by a reliable company using the electrolytic method, and clearly labeled with specifications such as FAC concentration.
(3) Hypochlorous acid is a very unstable substance, and when exposed to high heat or strong light, it may completely decompose and become ineffective in a short time. It is very dangerous to believe and use something that has already lost its effect. Handle HAW as having an expiration date, and if you do not use it for a while, put it in a closed container and store it in a cool and dark place. (See About saving HAW below)
(4) Please be aware that the efficacy of HAW depends on its usage and FAC concentration. The active ingredient of HAW is hypochlorous acid (HClO), and its efficacy can be measured as the FAC concentration. The higher the FAC concentration, the higher the sterilization ability. But if it is too high, it will be more irritating to the skin.
(5)Hypochlorous acid is consumed quickly in places where there is a large amount of organic matter such as food residue, so when sanitization such a place with HAW, it is necessary to wash the organic matter first. Of course, if the organic matter can be easily washed off with water, a large amount of HAW can be used for cleaning and sanitization at once.
(6)Roughly speaking, HAW may be used properly according to its FAC concentration as follows:
| FAC concentration | Usage example |
| 10-35mg/L | Washing vegetables |
| 35-60mg/L | Cleaning and sanitization of hands |
| 60-80mg/L | Cleaning and sanitization of cooking utensils |
(7)HAWs made by neutralizing sodium hypochlorite with acid are also on the market, but of course, they do not meet the standards for food additives set by MHLW. Some of them have FAC concentrations of well over 100 mg/L. Such products can be used in places that require strong sterilizing power, such as disinfecting toilet bowls. However, the higher the FAC concentration, the stronger the irritation to the skin, so care must be taken not to handle them with bare hands.

Effective storage period and storage method of HAW
Decomposition of hypochlorous acid
We explained that when hypochlorous acid comes into contact with an organic substance that is easily oxidized, it decomposes like HClO → HCl + O and gives oxygen to the substance. But, even if such a substance is not nearby, decomposition of HAW is always happening. When HAW decomposes, if there is no partner to give oxygen, the reaction may proceed in the opposite direction and immediately rejoin to hypochlorous acid. However, in another case, two oxygen atoms generated at the same time may combine to form an oxygen molecule such as 2HClO → 2HCl + O2. As this reaction progresses slowly, HAW becomes less effective* as it reduces its FAC concentration over time. It is unavoidable, but the validity period of HAW varies greatly depending on the storage environment, so we will explain how to store HAW. * Remember that chlorine in HCl is not FAC.
As factors that influence the expiration of HAW, Ishihara, et al.(2017) demonstrated:
The factors that promote the decomposition of hypochlorous acid are physical stimuli such as strong light, air contact, and high temperature, as well as extra chemical components in HAW such as organic compounds and various ions.
Effects of high temperature and strong light
It is said that the rate of any chemical reaction doubles when the temperature rises by 10℃. Since the FAC concentration of HAW decreases due to a chemical reaction, the effective period is doubled when stored at 20℃ and quadrupled when stored at 10℃ compared to when stored at 30℃.
As an example of the decomposition reaction of hypochlorous acid promoted by strong light and high temperature, there is a report that the FAC concentration became zero in just one day when exposed to direct sunlight in the summer(Yamaguchi pharmaceutical Association, 2020; in Japanese).
Importance of lid
Hypochlorous acid molecules are weakly volatile and slowly escape into the air. And it is said that, in the air, half of them decompose in just a few hours. In addition, the chemical reaction formula 2HClO → 2HCl + O2 written above shows that hypochlorous acid decomposes into hydrogen chloride and oxygen, and the oxygen also escapes into the air. As the process progresses, hypochlorous acid and oxygen in the air increase making it more and more difficult for them to escape from the water in a closed container. However, in an open container, the concentrations of hypochlorous acid and oxygen in the air do not increase, so they continue to escape into the air and the concentration of hypochlorous acid in the water decreases steadily. In other words, leaving the container lid open shortens the life of hypochlorous acid water.
Figure 4: Schematic image showing the importance of lid.
Left: With no lid, the gases of hypochlorous acid and oxygen escape steadily, and the concentration of hypochlorous acid in the water continues to decrease.
Right: With a lid, when hypochlorous acid and oxygen in the air inside the container reach certain concentrations, the rate of evaporation from water and the rate of dissolution into water become equal, and the decrease in hypochlorous acid concentration due to evaporation stops.
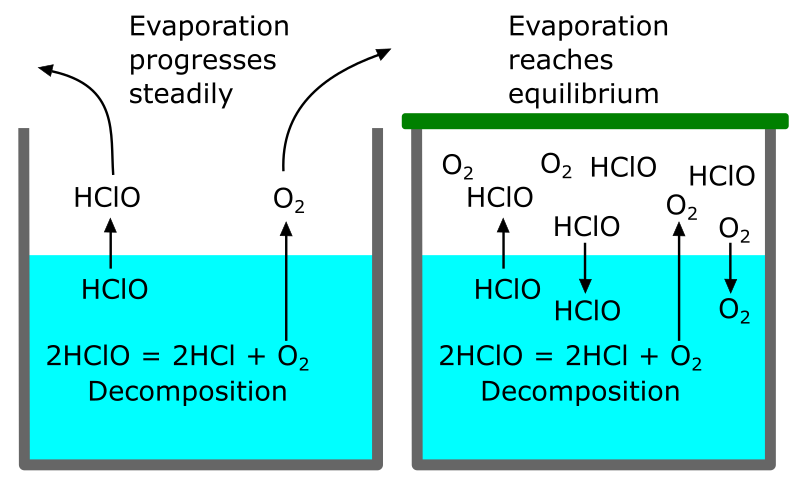
Regarding the importance of the lid, we have the following experimental results from Kitami Institute of Technology:
- When a HAW sample with a FAC concentration of 30 mg/L was placed in a colorless and transparent glass bottle and stirred under fluorescent light irradiation without the lid, a significant decrease in the FAC concentration was observed after 3 hours, and the concentration became zero after 15 hours.
- When agitated in the same bottle with a tight stopper, no significant decrease in concentration was observed even after 15 hours.
An example of less extreme adverse conditions is found in the research report published under the joint name of Obihiro University of Agriculture and Veterinary Medicine and ACT Corp. According to the report, when the container (light-shielding bottle) was left open in the laboratory, the FAC concentration of 100 mg/L decreased to 23 mg/L after leaving it for 17 days and to 2 mg/L after leaving it for 31 days.
Summary of how to store HAW
According to our experience, in the case of HAW with a well-controlled manufacturing process and good quality (no extra ingredients), the FAC concentration dropped from 40mg/L to about 30mg/L in 2 months when it was placed in a closed container and stored in a dark place at a temperature of about 10℃. Therefore, as long as the storage conditions are good, it can be considered that HAW with a FAC concentration of 50 mg/L at the time of shipment retains its sterilizing ability for about 2 or 3 months.
It is also known that if the environmental conditions such as temperature are the same, the higher the concentration of hypochlorous acid, the faster the decomposition reaction.
From the above, the important conditions for long-term storage of HAW can be summarized as:
- Prepare HAW with a FAC concentration you want to use(or slightly higher).
- Put it in a closed container that does not allow gases to pass through in a nearly full state.
- Keep out of light
- Keep in a quiet place at low temperature
Additional notes
We experienced that, after putting HAW in a spray bottle and leaving it for a while, the FAC concentration of the first sprayed HAW is lower than that in the container. Probably, the FAC concentration of hypochlorous acid remaining in the spray head decreased because the spray head has a lower ability to shield gas than regular bottle lids. Therefore, it is better to think that the first blow of HAW from a spray bottle that has been left for a certain period has a low sterilizing effect.
Even with the utmost care, long-term stored HAW may become ineffective for some reason. If you do not realize it and feel relieved that you have sterilized your surroundings with the HAW, the consequences can be ridiculous.
Therefore, if you want to use HAW after long-term storage for important purposes, for example, for cleaning and sterilizing hands as a countermeasure against the new coronavirus*, we recommend you to check the FACe concentration with a commercially available measuring instrument or test paper.
Select one that is suitable for measurement with FAC concentrations (note that it is different from the salinity) of about 10 to 80 mg/L.
Here is an example of inexpensive test strips for personal use to roughly measure FAC concentrations of HAW.
* According to the report of NITE, HAW with a FAC concentration of 35 mg/L or more is effective for CoVID-19.

Three types of electrolyzers for producing HAW
HAWs designated as food additives by MHLW are produced by electrolyzing saline solution or dilute hydrochloric acid. Due to the instability of hypochlorous acid as mentioned above, HAW is expected to be used immediately after manufacture in the equipment. There are three types of electrolyzers for manufacturing HAW: one no ion exchange diaphragm (one-chamber type), one with one diaphragm (two-chamber type), and one with two diaphragms (three-chamber type).
Do you remember the water electrolysis experiment you did in your school class? Before starting the experiment, a small amount of sodium hydroxide(NaOH) should have been added to the water to reduce the electrical resistance of the water. Then, if sodium chloride (NaCl) is dissolved in water instead of sodium hydroxide, what will happen when this solution is electrolyzed?
What happens around the electrodes
In this case, sodium ions(Na+) are attracted near the cathode, and excess sodium ions deprive water molecules of hydroxide ions (OH-), and hydrogen ions (H+) that have lost their partners obtain electrons from the cathode. As a result, hydrogen molecules (H2) and sodium hydroxide (NaOH) are generated around the cathode. Since hydrogen molecules are hardly soluble in water, they escape into the air, and only sodium hydroxide remains in the solution.
2Na+ + 2H2O → 2NaOH + 2H+
2H+ + 2e- → H2 (e is an electron)
On the other hand, at the anode, the attracted chlorine ions (Cl-) are deprived of electrons by the anode to generate chlorine molecules (Cl2). Since the chlorine molecules are highly reactive, they immediately react with water molecules, resulting in the generation of hydrogen chloride (HCl) and hypochlorous acid (HClO).
2Cl- → Cl2 + 2e-H2O + Cl2 → HCl + HClO
A reaction that forms oxygen molecules also occurs.
4OH- → 2H2O + O2
But the amount of oxygen generated is small. This is because chlorine ions are far more likely to be deprived of electrons by the anode than hydroxide ions.
Do you understand how hypochlorous acid is produced by the electrolysis of saline solution?
One-chamber electrolyzer
Comprehensively looking at the whole process in which the reactions to make acid and base are proceeding at the same time, the overall reaction can be expressed by the following formula.
2NaCl + 3H2O → 2NaOH + HCl + HClO + H2
The hydrogen molecules (H2) generated here quickly escape into the air. Finally, when the power of the device is turned off, if there is no partition between the two electrodes, the entire electrolyzed water will mix. Then, one molecule of sodium hydroxide (NaOH) generated on the cathode side reacts with one molecule of hydrogen chloride (HCl) generated on the anode side to form water and sodium chloride.
NaOH + HCl → NaCl + H2O
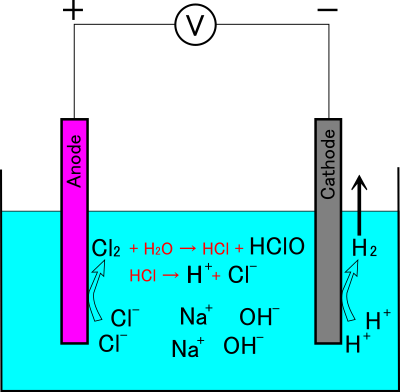
As a result, the electrolyzed water produced becomes alkaline because the three components remaining in the solution are hypochlorous acid, sodium hydroxide, and sodium chloride. In other words, the balance of the whole substance when the saline solution is electrolyzed by the one-chamber electrolyzer is as follows(Figure 5).
NaCl + 2H2O → NaOH + HClO + H2
Figure 5: Schematic image showing chemical reactions occurring in a one-chamber electrolyzer.
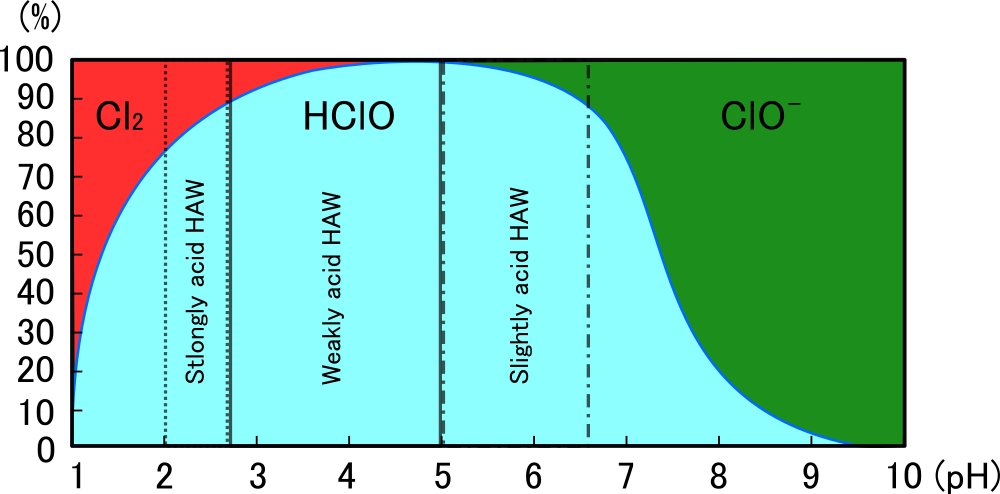
Now, look at the figure and description below. This figure shows that the higher the pH, the higher the rate at which hypochlorous acid dissociates into hydrogen ions (H+) and hypochlorite ions (ClO-). As mentioned above, the sterilizing power of hypochlorous acid molecules is said to be about 80-100 times that of hypochlorite ions, so the sterilizing power is greatly reduced in alkaline solutions.
For this reason, to obtain the "slightly acidic hypochlorous acid water produced by a one-chamber electrolyzer" defined by MHLW, it is necessary to electrolyze dilute hydrochloric acid instead of (or in addition to) the sodium chloride solution. By doing so, the pH of the produced HAW can be adjusted to be between 5 and 6.5.
Figure 6: PH dependence of the abundance ratio of the FAC components in HAW, and pH ranges of the HAWs defined as food additives by MHLW.
Modified after various sources.
This figure shows that when the hydrogen ion concentration [H +] is low (pH is high), the reaction HClO → H+ + ClO- progresses and the proportion of hypochlorite ions increases, and when the hydrogen ion concentration increases, the reaction H+ + 2HClO → Cl2 + 2H2O progresses and the proportion of chlorine molecules increases.
Two-chamber electrolyzer
In the two-chamber electrolyzer, the electrodes are separated by a diaphragm that allows only ions to pass through (the figure shows a bipolar ion exchange membrane that allows both cations and anions to pass through). In this device, as electrolysis progresses, chloride ions are consumed around the anode, hydrogen chloride and hypochlorous acid are concentrated, and sodium ions corresponding to the consumed chloride ions move to the cathode side through the diaphragm.
Figure 7: Schematic image showing chemical reactions occurring in two-chamber electrolyzer with bipolar ion exchange membrane.
The bipolar ion exchange membrane allows both anions and cations to pass through.
Due to the potential difference between the electrodes, chloride ions tend to move to the anode side and sodium ions to the cathode side.
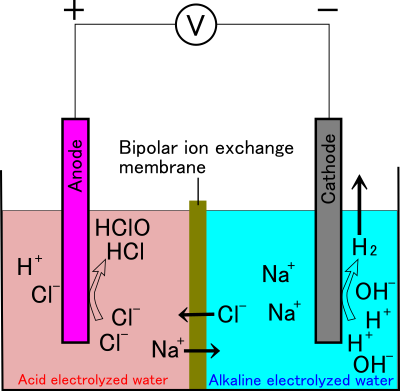
At around the cathode, hydrogen ions are consumed and sodium hydroxide is concentrated, and most of the generated hydrogen is released into the atmosphere. As a result, two types of electrolyzed water, acidic and alkaline, are obtained, and the acidic electrolyzed water corresponds to HAW. However, the reaction does not proceed until all the NaCl is consumed, and the NaCl remains in both of the electrolyzed water.
Three-chamber electrolyzer
The three-chamber electrolyzer separates the electrodes with two diaphragms. The device is activated with the NaCl solution in the central chamber and pure water in the other two chambers. In this device, the diaphragm on the cathode side allows only cations to pass through, and the other allows only anions to pass through. In other words, sodium ions can move only to the cathode side, and chloride ions to the anode side.
Figure 8: Schematic image showing chemical reactions occurring in a three-chamber electrolyzer.
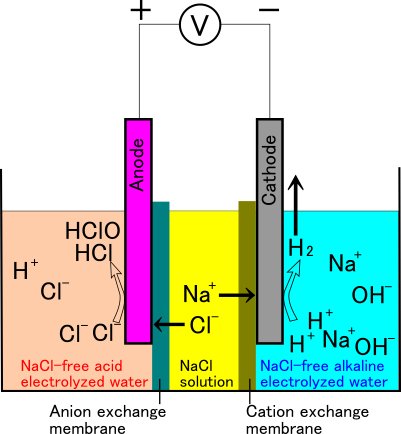
With this device, acidic electrolyzed water is obtained on the anode side and alkaline electrolyzed water is obtained on the cathode side as the electrolysis progresses. In this respect, it is similar to the two-chamber type electrolyzer, but there are no sodium ions on the anode side (acidic electrolyzed water) and no chloride ions on the cathode side (alkaline electrolyzed water). Therefore, "salt-free HAW" can be obtained.
The HAW manufacturing equipment "CLEAN·FINE" provided by ACT Corp. corresponds to this type.
If you want to know more about CLEAN·REFRE, please read "CLEAN·REFRE, a NaCl-free hypochlorous acid water"

